page 1

page 2

page 3

page 4

page 5

page 6

page 7

page 8

page 9

page 10

page 11

page 12

page 13

page 14

page 15

page 16

page 17

page 18

page 19

page 20

page 21

page 22

page 23

page 24

page 25

page 26
REAGENTS
SODIUM ACETATE s-aqueous
Official edition
IPK PUBLISHING STANDARDS Moscow
UDC 547.292433-41:006.354 Group L52 STATE STANDARD OF THE UNION OF THE SSR
Reagents
SODIUM ACETATE 3-WATER Specifications
Reagents Sodium acetate, 3 aqueous Specifications
OKP 26 3421 1290 05
Introduction date 01.01.79
This standard applies to sodium acetate, 3-aqueous, which is colorless transparent crystals, soluble in water, erodes when dry and deliquescent when humid air
Formula CH 3 COONa ZN 2 0
Relative molecular weight (according to international atomic masses 1987) - 136.08
It is allowed to manufacture 3-aqueous sodium acetate according to Appendix 1 and conduct analyzes according to Appendix 2
1. TECHNICAL REQUIREMENTS
1 1 3-aqueous sodium acetate must be manufactured in accordance with the requirements of this standard according to the technological regulations approved in the prescribed manner
1 2 In terms of physico-chemical parameters, 3-aqueous sodium acetate must comply with the standards specified in Table 1
Official publication Reprint prohibited
© Standards Publishing House, 1978 © IPK Standards Publishing House, 1997 Reissued with Amendments
for the drug pure - 0.010 mg Mg,
0.2 cm 3 titanium yellow solution and 2 cm 3 hydroxide solution
In case of disagreement in the assessment of the mass fraction of magnesium, the determination is carried out by the atomic absorption method.
3.11a. Determination of the mass fraction of calcium and magnesium (atomic absorption method)
3.1 la. 1. Equipment, glassware, reagents and solutions
Spectrophotometer "Saturn" or another with similar metrological characteristics.
Acetylene dissolved technical according to GOST 5457.
Compressed air for power supply of instrumentation.
Dilute each solution to the mark with water and mix thoroughly.
|
table 2 |
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
3 11a 4 Analysis
The mass fraction of calcium is determined in the emission mode using the analytical line 422.7 nm
At least two weighed portions of the drug are taken for analysis. After preparing the device in accordance with the instructions for use attached to it, photometry of the analyzed solution and reference solutions is carried out in order of increasing impurity content. Then photometry is carried out in reverse order and the arithmetic mean reading is calculated for each solution, taking into account as correction reading obtained by photometry of the first reference solution After each measurement, water is sprayed
The mass fraction of magnesium is determined according to GOST 22001 3 11a 5 Processing of results
Based on the data obtained, a calibration graph is built for reference solutions, plotting the radiation intensity on the ordinate axis, the mass fraction of calcium impurities in terms of the drug - on the abscissa axis
The mass fraction of calcium in the preparation is found according to the schedule The result of the analysis is taken as the arithmetic mean of the results of two parallel determinations, the relative discrepancy between which does not exceed the permissible discrepancy equal to 20% The permissible relative total error of the analysis result is ± 10% with a confidence probability P - 0.95
S. 12 GOST 199-78
The mass fraction of magnesium in the preparation is found according to GOST 22001.
3.11a.2-3.11a.5. (Introduced additionally, Amendment No. 1).
3.12. Determination of the mass fraction of arsenic The determination is carried out according to GOST 10485. At the same time, 2.00 g of the drug is placed in the flask of the device for the determination of arsenic, dissolved in 30 cm 3 of water, and then the determination is carried out by the method using mercury bromide paper in a sulfuric acid medium.
The drug is considered to comply with the requirements of this standard if the color of mercury-bromine paper when interacting with the analyzed solution is not more intense than the color of mercury-bromine paper when interacting with a solution prepared simultaneously with the analyzed one and containing in the same volume: for the preparation, pure for analysis - 0.001 mg As,
20 ml of sulfuric acid solution, 0.5 ml of 2-aqueous tin (II) chloride solution and 5 g of zinc.
(Revised edition, Rev. No. 2).
3.13. Determination of the mass fraction heavy metals
Type and type of container: 2-1, 2-2, 2-4, 2-9.
Handling signs "Top" and "Fragile" Caution" are applied to the shipping container (for glass consumer containers) in accordance with GOST 14192
4 2 The drug is transported by all means of transport in accordance with the rules for the carriage of goods in force on this type of transport
4 3 The drug is stored in the manufacturer's packaging in covered warehouses
5. MANUFACTURER WARRANTY
5 1 The manufacturer guarantees the compliance of 3-aqueous sodium acetate with the requirements of this standard, subject to the conditions of transportation and storage
5 2 Guarantee period storage of the drug - three years from the date of manufacture
5 1.52 (Revised edition, Rev. No. 1).
6. SAFETY REQUIREMENTS
6 1 3-aqueous sodium acetate irritates the skin and mucous membranes of the eyes and respiratory tract
6 2 When working with the drug, you should use products personal protection
(Revised edition, Rev. No. 2).
6 3 Premises in which work with the drug is carried out must be equipped with continuously operating supply and exhaust ventilation
The analysis of the drug should be carried out in a laboratory fume hood.
(Changed edition, Rev. No. 1, 2).
S. 14 GOST 199-78
ANNEX 1 Mandatory
ISO 6353-2-82 Reagents for chemical analysis. Part 2. Specifications. First episode
Relative molecular weight - 136.08
P29I Technical requirements Mass fraction of 3-aqueous sodium acetate CH3COONa ZH2O, %, not less than 99
pH of solution with mass fraction 5 % 7,5-9
Mass fraction of chlorides, %, no more than 0.0005
Mass fraction of phosphates, %, no more than 0.0005
Mass fraction of sulfates, %, no more than 0.002
Mass fraction of aluminum, % no more than 0.0005
Mass fraction of calcium, %, no more than 0.002
Mass fraction of copper, %, no more than 0.0005
Mass fraction of iron, %, no more than 0.0005
Mass fraction of lead, %, no more than 0.0005
Mass fraction of potassium, %, no more than 0.005
Mass fraction of substances that reduce KM11O4 in terms of HCOOH, %, not more than 0.005
R29. Sodium acetate 3-aqueous CH3 COONa ZH2O
P292 Preparation of test solution Dissolve 20 g of sample in 100 ml of water and dilute to 200 ml P 29 3 Perform analysis
R 29 3 1 Determination of the mass fraction of 3-aqueous sodium acetate Approximately 0.4 g of the sample is weighed to the nearest 0.0001 g and dissolved in 25 cm 3 acetic acid
To the resulting solution, add 0.4 cm 3 of an acetic acid solution of 1-naphthol benzene a with a mass fraction of 0.1% and titrate with a solution of perchloric acid of a molar concentration exactly c (NSO 4) = 0.1 mol / dm 3, in the presence of acetic acid to appearance of green color
1.00 cm 3 of a solution of perchloric acid of molar concentration c (HC1C> 4) \u003d \u003d 0.100 mol / dm 3 corresponds to 0.01361 g CH 3 COONa ZH2O R 29 3 2 Determination of the pH of a solution with a mass fraction of 5%
The determination is carried out in accordance with OM 31 G using a glass indicator electrode
R 29 3 3 Determination of the mass fraction of chlorides
Take 20 ml of the test solution (R 29 2) and analyze in accordance with OM 2 *, using instead of a solution of nitric acid with a mass fraction of 25% 1 cm 3 nitric acid
Prepare a control solution using 1 cm3 of reference chloride solution II (1 cm3 = 0.0005% C1)
Comparative chloride solution II is prepared immediately before use by diluting the stock solution in a volumetric flask in a ratio of 1 100 Basic
the mixture is prepared as follows 1.65 g NaCl P 29 3 4 Determination of the mass fraction of phosphates
Take 20 cm 3 of the test solution (P 29 2), add 50 cm 3 of water and analyze in accordance with OM 4 *
Prepare a control solution using 1.5 cm 3 of phosphate reference solution II (1.5 cm 3 = 0.0005% P0 4)
Phosphate reference solution I is prepared immediately before use by diluting the stock solution in a volumetric flask in a ratio of 1 100. The stock solution is prepared as follows: 1.43 g KH 2 P0 4
dissolve in water, dilute with water to the mark in a volumetric flask with a capacity of 1000 cm 3 and mix
P 29 3 5 Determination of the mass fraction of sulfates
Take 20 ml of test solution (P 29 2) and analyze according to OM 3*
Prepare a control solution using 4 cm 3 of sulfate reference solution II (4 cm 3 = 0.002% S0 4)
Sulfate reference solution II is prepared immediately before use by diluting the stock solution in a volumetric flask in a ratio of 1 100. The stock solution is prepared as follows: 1.81 g K 2 S0 4
dissolve in water, dilute with water to the mark in a volumetric flask with a capacity of 1000 cm 3 and mix
P 29 3 6 Determination of the mass fraction of aluminum
Take 15 ml of the test solution (P 29 2) and analyze according to OM 9*
Prepare a control solution using 5 cm 3 of the test solution (P 29 2) and 0.5 cm 3 of the aluminum-containing reference solution II (0.5 cm 3 \u003d - 0.0005% A1)
General methods of analysis (GM) - according to ISO 6353-1-82
dilute in will, dilute with water to the mark in a volumetric flask with a capacity of 1000 cm 3 and mix
R 29 3 7 Determination of the mass fraction of a substance
The determination is carried out by atomic absorption spectroscopy in accordance with OM 29* under the following conditions.
R 29 3 8 Determination of the mass fraction of copper and lead
Ag/AgCl, saturated with KS!
0.75 V Cu +0.185 V Pb: -0.31 V Additive method
The determination is carried out by the method of anodic dissolving voltammetry in accordance with OM 33 * using a solution of 1 g of the sample in 25 cm 3 of a sulfuric acid solution with a molar concentration of exactly c (1/2 H 2 S0 4) ~ = 1 mol / dm 3
Working electrode.....
Reference electrode. .
initial potential
Peak Potentials
Definition
P 29 3 9 Determination of the mass fraction of iron
Take 25 cm 3 of the test solution (P 29 2), neutralized with a solution of of hydrochloric acid with a mass fraction of 25%, and analyzed in accordance with OM 8.1 *.
Prepare a control solution using 5 cm 3 of the test solution (P 29 2) and 1 cm 3 of the iron-containing reference solution II (1 cm 3 ™ 0.0005% Fe).
The iron-containing comparison solution II is prepared immediately before use by diluting the stock solution in a volumetric flask in a ratio of 1: 100. The stock solution is prepared as follows, a mixture of 8.63 g NH 4 Fe (S0 4) 2 12H2O and 10 cm 3 H2S0 4 solution with a mass fraction 25% is dissolved in water, diluted with water to the mark in a volumetric flask with a capacity of 1000 cm 3 and mixed.
R 29.3.10. Determination of the mass fraction of potassium
General methods of analysis (GM) - according to ISO 6353-1-82
The determination is carried out by flame photometry in accordance with OM 30* under the following conditions.
R 29 3 11 Determination of the mass fraction of substances that reduce KMp0 4
Dissolve 10 g of the sample in 100 cm 3 of water, add 1 cm 3 of a solution of potassium permanganate with a molar concentration of exactly 1/5 KMn04 = = 0.1 mol / dm 3, boil for 5 minutes and cool
The pink color of the solution should not disappear completely Add 2 g of potassium iodide and 20 cm 3 of a solution of sulfuric acid with a mass fraction of 20 ° L and titrate with a solution of sodium sulphate with a molar concentration exactly c (No. 2826) 3) = 0.1 mol / dm 3 to discoloration of the solution At the same time, a control determination is carried out using the same amounts of all reagents, except for a solution of sodium sulphate of exact concentration, excluding the analyzed sample
The volume of the required titrant, after taking into account the control experience, should not exceed 0.25 cm 3 .
APPENDIX 2 Mandatory
ISO 6353-1-82 Reagents for chemical analysis Part 1: General test methods
5.2. Determination of the mass fraction of chlorides (OM 2) The specified volume of the test solution (R 29.3.3) is acidified with 1 cm 3 of a solution of nitric acid with a mass fraction of 25% and 1 cm3 of a solution of silver nitrate with a mass fraction of approximately 1.7% is added
After 2 minutes, compare the opalescence of the analyzed solution with that of the control solution.
General methods of analysis (GM) - according to ISO 6353-1-82
53 Determination of the mass fraction of sulfates (OM 3) About 25 cm 3 of a solution of potassium sulfate with a mass fraction of 0.02% in ethanol with
30% volume fraction is mixed with 1 cm 3 of a solution of barium chloride dihydrate with a mass fraction of 25% (seed solution) Exactly after 1 minute, the indicated volume of the test solution (P 29 3 5), previously acidified with 0.5 cm 3 of hydrochloric solution, is added to this mixture acids with a mass fraction of 20%
The mixture is allowed to stand for 5 minutes and its turbidity is compared with the turbidity of the mixture obtained by similar processing of the corresponding control solution.
54 Determination of the mass fraction of phosphates (OM 4) 5 cm 3 of a solution of ammonium molybdate with a mass fraction of 10% is added
to the indicated volume of the test solution (P 29 3 4) Adjust the pH of the solution to 1.8 and heat to a boil Cool, add 12.5 cm 3 of a hydrochloric acid solution with a mass fraction of 15% and extract with 20 cm 3 of diethyl ether The organic layer is washed with hydrochloric acid acids with a mass fraction of 5% and restore the molybdenum phosphate complex by adding 0.2 cm 3 of a solution of 2-aqueous tin (II) chloride with a mass fraction of 2% in hydrochloric acid solution Compare the color intensity of the obtained organic layer with the color intensity of the organic layer obtained by similar treatment appropriate control solution
58 Determination of the mass fraction of iron (OM 8 1) The determination is carried out using 1,10-phenanthroline, following the instructions of GOST 10555
59 Determination of the mass fraction of aluminum (OM 9) 5 9 1 Preparation of the aluminon reagent
0.25 g of aluminone (ammonium salt of auryl tricarboxylic acid) and 5 g of gum arabic are added to 250 cm 3 of water and heated until dissolved. 87 g of ammonium acetate are dissolved in the resulting solution
Then add 145 cm 3 of a solution of hydrochloric acid with a mass fraction of 15% and dilute with water to a volume of 500 cm 3 If necessary, the solution is filtered The solution is valid for a month 5 9 2 Method of analysis
A sample with an indication of the volume of the test solution (R 29 3 6) is neutralized with litmus, 1 cm 3 of an acetic acid solution with a mass fraction of 30% is added and the pH of the solution is adjusted to 4.5 with an ammonia solution with a mass fraction of 10% Then 0, 1 cm 3 thioacetic acid and 3 cm 3 aluminone reagent (p 5 9 1), heated at a temperature of about 100 ° C for 10 minutes and cooled
Compare the intensity of the red color with the intensity of the color of the solution obtained by similar processing of the corresponding control solution.
GOST 199-78 S. 19
5 29 Atomic absorption spectroscopy (OM 29)
5 29 1 General notes
The test sample or its solution is sucked into a high-temperature flame created by a mixture of combustible gas and an oxidizing gas, which allows the test sample to evaporate and dissociate its molecules into atoms. A device with flameless heating can be used. a tube activated by microwave radiation produces radiation with a wavelength corresponding to the excitation energy of the atoms of the substance under test The atoms of the analyte absorb a certain proportion of this radiation, proportional to their amount in the ground (unexcited) state, and this absorption is recorded by a suitable atomic absorption spectrometer 5 29 2 Method analysis
The essence of the method, the variety of existing devices, the abundance of parameters associated with the test sample and the device, and the multiplicity of influencing factors do not allow us to give detailed instructions
The choice of procedure is determined by the required degree of accuracy. Consideration should be given to the possibility of interference from flame and flameless heat sources. If the instrument is equipped with a flame heat source, the determination is carried out using aqueous solutions of the test substances slightly acidified with nitric or hydrochloric acid. In order to take into account the effects of the solution, it is recommended to use the addition method. This method consists in that the determination is carried out on a series (the size of which depends on the required accuracy, but not less than two) aliquots of the test solution, to which known amounts of the analyte are added.
Wavelengths corresponding to the resonance lines and other special information are given in the descriptions relating to a particular specific reagent.
5 30 Flame photometry (OM 30)
5 30 1 General information
This method is based on the measurement of the intensity of light emitted by some atoms as they transition from an excited state to a state of lower energy Atoms become excited in a flame created by a mixture of combustible gas and an oxidizing gas The intensity of radiation emitted by atoms is measured using a suitable photometric system with a monochromator or with filters
Note Different mixtures of flame gases than those specified in the descriptions may be used, and it may be necessary to change the concentrations of solutions recommended in the same descriptions accordingly.
|
Table 1 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
(Revised edition, Rev. No. 1).
2. ACCEPTANCE RULES
2.2. Mass fraction of sulfates, phosphates, aluminum, calcium,
5 30 2 Method of analysis
The procedure is similar to that of atomic absorption spectroscopy Conditions for each specific analysis can be found in the descriptions relating to the analyzed reagent 5 311 Determination of pH (OM 311)
5 3111 General provisions
Consider a galvanic cell reference electrode - saturated solution of CO - solution of R / pt H 2
For buffer solutions R\ and R 2 with known pH values, respectively pHd! and pH/? 2 , the measured values of the potential difference are, respectively, Ej and £ 2
If the solution R in the considered galvanic cell is replaced by the test solution with unknown pH, then the pH of the test solution can be calculated from the difference in the measured potential values
If all measurements are carried out at the same temperature and at a constant concentration of a potassium chloride solution, the pH of the test solution can be calculated using the following formulas
E\ - £ u ^ -^- + pH / g y,
-^- + pH/? 2,
where £ u is the electromotive force of the galvanic cell with the test solution,
S - slope
pH L/ -pH/g 2
5 31 1 2 Equipment
pH meter with a glass electrode connected to a millivoltmeter with high resistance and with a scale calibrated in pH units Such a device, registering the potential difference between a pH-sensitive electrode (glass, antimony) and a reference electrode connected by an electrolytic bridge (for example, a saturated KC1 solution ), makes it possible to read the pH value directly from the scale
5 3113 Calibration
The pH meter is calibrated using buffer solutions of known hydrogen ion activity, e.g.
a) oxalate buffer solution,
b) tartrate buffer solution,
magnesium, arsenic and heavy metals the manufacturer determines periodically in every 20-batch
(Introduced additionally, Rev. No. 1).
3. METHODS OF ANALYSIS
Weighing using laboratory scales general purpose types VLR-200g, VLE-200g or VLKT-500g-M
It is allowed to use other measuring instruments with metrological characteristics and equipment with technical specifications no worse, as well as reagents and materials in quality not lower than those specified in this standard
3 1, 3 2a (Revised edition, Rev. No. 2).
32 Determination of the mass fraction of 3-aqueous sodium acetate
Filtering crucible TF-POR 10(16) XC according to GOST 25336.
3.3.2. Conducting an analysis
100.00 g of the drug is placed in a glass and dissolved in 400 cm 3 of water. The beaker is covered with a watch glass or a cup and incubated for 1 hour in a water bath.
Then the solution is filtered through a filter crucible, previously dried to constant weight and weighed, the result
Weighings in grams are recorded to the fourth decimal place. The filter residue is washed with 150 cm 3 of hot water and dried in an oven at 105-110°C to constant weight.
The preparation is considered to comply with the requirements of this standard if the mass of the residue after drying does not exceed:
for the drug pure for analysis - 2 mg,
for the drug pure - 5 mg.
Permissible relative total error of the analysis result is ±30% at a confidence level P = 0.95.
3.3.1. 3.3.2. (Changed edition, Rev. No. 1,2).
3.4. Determination of the mass fraction of acids in terms of acetic acid and the mass fraction of alkalis in terms of sodium hydroxide
(Revised edition, Rev. No. 1).
3.4.1. Glassware, reagents and solutions
Burette with a capacity of 5 cm 3 and a division value of 0.02 cm 3.
Distilled water, not containing carbon dioxide; prepared according to GOST 4517.
Phenolphthalein (indicator), alcohol solution with a mass fraction of 1%; prepared according to GOST 4919.1.
3.4.2. Conducting an analysis
10.00 g of the drug is placed in a 100 cm 3 conical flask, dissolved in 50 cm 3 of water and three drops of phenolphthalein solution are added. If the solution remains colorless, then sodium hydroxide solution is added dropwise from the burette until a pink color appears. If the solution is colored pink, then add hydrochloric acid solution drop by drop from the burette until the pink color disappears.
The mass fraction of acids in terms of acetic acid (CH 3 COOH) or the mass fraction of alkalis in terms of sodium hydroxide (NaOH) (X) as a percentage is calculated by the formula
where V is the volume of a solution of sodium hydroxide with a molar concentration of exactly 0.1 mol / dm 3 or a solution of hydrochloric acid with a molar concentration of exactly 0.1 mol / dm 3 used for titration, cm 3, t is the weight of the sample of the preparation, g,
m x is the mass of CH 3 COOH (0.006) or NaOH (0.004), corresponding to 1 cm 3 of a solution of sodium hydroxide with a molar concentration of exactly 0.1 mol / dm 3 or a solution of hydrochloric acid with a molar concentration of exactly 0.1 mol / dm 3, g Za the result of the analysis is taken as the arithmetic mean of the results of two parallel determinations, the absolute discrepancy between which does not exceed the permissible discrepancy equal to 0.004% Rev. No. 2).
35 Determination of the mass fraction of sulfates The determination is carried out according to GOST 10671 5 In this case, 2.00 g of the drug is placed in a conical flask with a capacity of 50 or 100 cm 3 (with a mark of 25 cm 3) and dissolved in 15 cm 3 of water. Then 0.1 cm 3 is added a solution of 2,4-dinitrophenol with a mass fraction of 0.1% (prepared according to GOST 4919 1) and drop by drop, stirring constantly, a solution of hydrochloric acid until the solution becomes colorless. water Then the volume of the solution is adjusted with water to 25 cm 3 and then the determination is carried out by phototurbidimetric or visual-non-phelometric (method 1) method
The drug is considered to comply with the requirements of this standard if the mass of sulfates does not exceed 0.02 mg pure for analysis for the drug, 0.04 mg for the pure drug
In case of disagreement in the assessment of the mass fraction of sulfates, the analysis is carried out by the phototurbidimetric method (Changed edition, Rev. No. 1).
36 Determination of the mass fraction of phosphates The determination is carried out according to GOST 10671 6 In this case, 10.00 g of pre-
paratha is placed in a cylinder with a ground stopper (GOST 1770) with a capacity of 50 cm 3 and dissolved in 7.5 cm 3 of a solution of nitric acid with a mass fraction of 50% The volume of the solution is adjusted with water to 15 cm 3, mixed and then the determination is carried out photometrically by the yellow color of the phosphorus-vanadium-molybdenum complex The drug is considered to comply with the requirements of this standard if the mass of phosphates does not exceed 0.02 mg pure for analysis for the drug, 0.10 mg for the pure drug
It is allowed to complete the determination visually with the extraction of the phosphorvanadium-molybdenum complex into layers of isoamyl alcohol (5 cm 3)
At the same time, under the same conditions and with the same amounts of reagents, a control experiment is carried out. If an admixture of phosphates is detected, the result of the analysis is corrected.
In case of disagreement in the assessment of the mass fraction of phosphates, the determination is completed photometrically
37 Determination of the mass fraction of chlorides The determination is carried out according to GOST 10671 7 In this case, 2.00 g of the drug is placed in a volumetric (when determined by the phototurbidimetric method) or a conical flask with a capacity of 100 cm 3 with a mark of 40 cm 3 (when determined by the visual nephelometric method) and dissolved in 30 cm 3 of water Then 3.5 cm 3 of a solution of nitric acid with a mass fraction of 25% is added, heated to a boil and cooled. fractions of 1% Further, the determination is carried out by a phototurbidimetric or visual-nephelometric method, without adding a solution of nitric acid. The preparation is considered to comply with the requirements of this standard if the mass of chlorides does not exceed pure for analysis for the preparation - 0.020. mg, for pure drug - 0.020 mg
GOST 4517.
39 Determination of the mass fraction of iron The determination is carried out according to GOST 10555 In this case, 5.00 g of the drug is placed in a glass with a capacity of 100 cm 3 and dissolved in 20 cm 3 of water. Then 6 cm 3 of a hydrochloric acid solution with a mass fraction of 25% (GOST 3118) is added, heated to a boil and boil for 2-3 minutes. The solution is cooled, carefully neutralized with aqueous ammonia (GOST 3760) according to universal indicator paper to pH 2, 0.1 cm 3 of acetic acid solution is added, and then the determination is carried out by the 1,10-phenanthroline method.
The drug is considered to comply with the requirements of this standard if the mass of iron does not exceed 0.010 mg pure for analysis for the drug, 0.025 mg for the pure drug
At the same time, under the same conditions and with the same amounts of reagents, a control experiment is carried out. If iron impurities are detected, the result of the analysis is corrected.
It is allowed to complete the determination visually. In case of disagreement in the assessment of the mass fraction of iron, the determination is completed photometrically.
310 Determination of the mass fraction of calcium (chemical method)
3 9, 3 10 (Revised edition, Rev. No. 1).
3 10 1 Glassware, reagents and solutions
Pipettes with a capacity of 1 or 2 cm3 and pipettes with a capacity of 5 or 10 cm3
Murexide, solution with a mass fraction of 0.05% (valid for 2
(Changed edition, Rev. No. 1, 2).
3 10 2 Analysis
0.60 g of the drug is placed in a test tube, dissolved in 5 cm 3 of water, 2 cm 3 of sodium hydroxide solution and 1 cm 3 of murexide solution are added, thoroughly mixing the solution after adding each reagent
The drug is considered to comply with the requirements of this standard if the pinkish-violet color of the analyzed solution observed in 1-2 minutes in transmitted light against the background of milk glass is not more intense than the color of the solution prepared simultaneously with the analyzed one and containing in the same volume:
for the drug pure for analysis - 0.009 mg Ca, for the drug pure - 0.015 mg Ca,
2 cm 3 sodium hydroxide solution and 1 cm 3 murexide solution. The color of the solution is stable for 10 minutes.
In case of disagreement in the assessment of the mass fraction of calcium, the determination is carried out by the atomic absorption method.
(Revised edition, Rev. No. 1).
3.11. Determination of the mass fraction of magnesium (chemical method)
(Revised edition, Rev. No. 1).
3.11.1. Glassware, reagents and solutions
Pipettes with a capacity of 1 and 2 cm 3 and pipettes with a capacity of 5 or 10 cm 3. Test tube P4-25-14/23 XC according to GOST 25336.
Titanium yellow, solution with a mass fraction of 0.05%. (Changed edition, Rev. No. 1, 2).
3.11.2. Conducting an analysis
2.00 g of the drug is placed in a test tube, dissolved in 6 cm 3 of water, the volume of the solution is adjusted to 8 cm 3 with water and mixed. Then 0.2 cm 3 of titanium yellow solution and 2 cm 3 of sodium hydroxide solution are added, thoroughly mixing the solution after adding each reagent.
The drug is considered to comply with the requirements of this standard if the pink color of the analyzed solution observed after 10 minutes along the axis of the test tube is not more intense than the color of the solution prepared simultaneously with the analyzed and containing in the same volume:
for the drug pure for analysis - 0.005 mg Mg,









Quality requirements
Peat resources Milling peat extraction
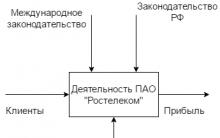
Closed Joint Stock Company Transtelecom
Setting up Wi-Fi equipment
Pasteurization drawer for the selection of "heads" during rectification